Our B2B Services



We bring ideas to life by combining years of experiences of our very talented team.
We Have
Manufacturers on our Panel




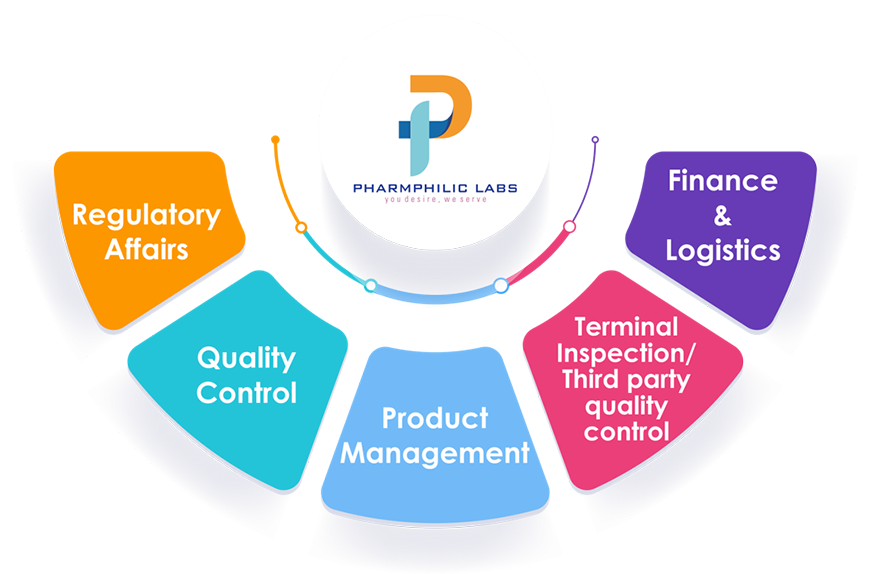
Our Process Flow
Product lifecycle management is the management of the entire life-cycle of the drug product from its development to the market authorization. During this period, several departments such as Regulatory Affairs, Quality Control, Product Management, Terminal Inspection / Third party Quality control, Finance & Logistics plays a vital role and their expertise are crucial for the product registration.
Our Core Values
We provide clients with fast track access to our pharmaceutical manufacturing partners in India that have the most advanced infrastructure, quality and manufacturing certifications.
We identify the most suitable vendor, validate their facilities, prepare and review dossiers specific to the needs of the country, manage quality and logistics till the client`s warehouse.
We make doing business with India easy and enjoyable. Our experts manage the various stakeholders in the entire supply chain providing to our client's a seamless experience.
We pride ourselves in our commitment to serving.

Who We Are ?
When it comes to ensuring drug registration in a country, regulatory affairs consultants are an excellent option. Choosing the right consultant can help your organization avoid problems related to registration and improve the time-to-market for your product.
Here we help organization to create a solid regulatory strategy and know how to: -
.- Plan and organize work effectively.
- Maintain confidentiality and security of information.
- Understand risks associated with incomplete regulatory submission of documents.
- Work with experienced team members in the field of Quality control/Quality Assurance/ Technology transfer/ Dossier compilation (ACTD, CTD, eCTD & REGIONAL).
- Implement best practices in regulatory writing.

